Migraine Treatments of the Month: The Anti-CGRP Monoclonal Antibodies
/Although we have discussed the anti-CGRP monoclonal antibodies (mABs) in a number of issues over the past several years, we’ve never devoted an entire article to this intriguing, important and now commonly prescribed class of medications. To correct this deficiency we designated the anti-CGRP mABs as this issue’s “migraine treatments of the month”
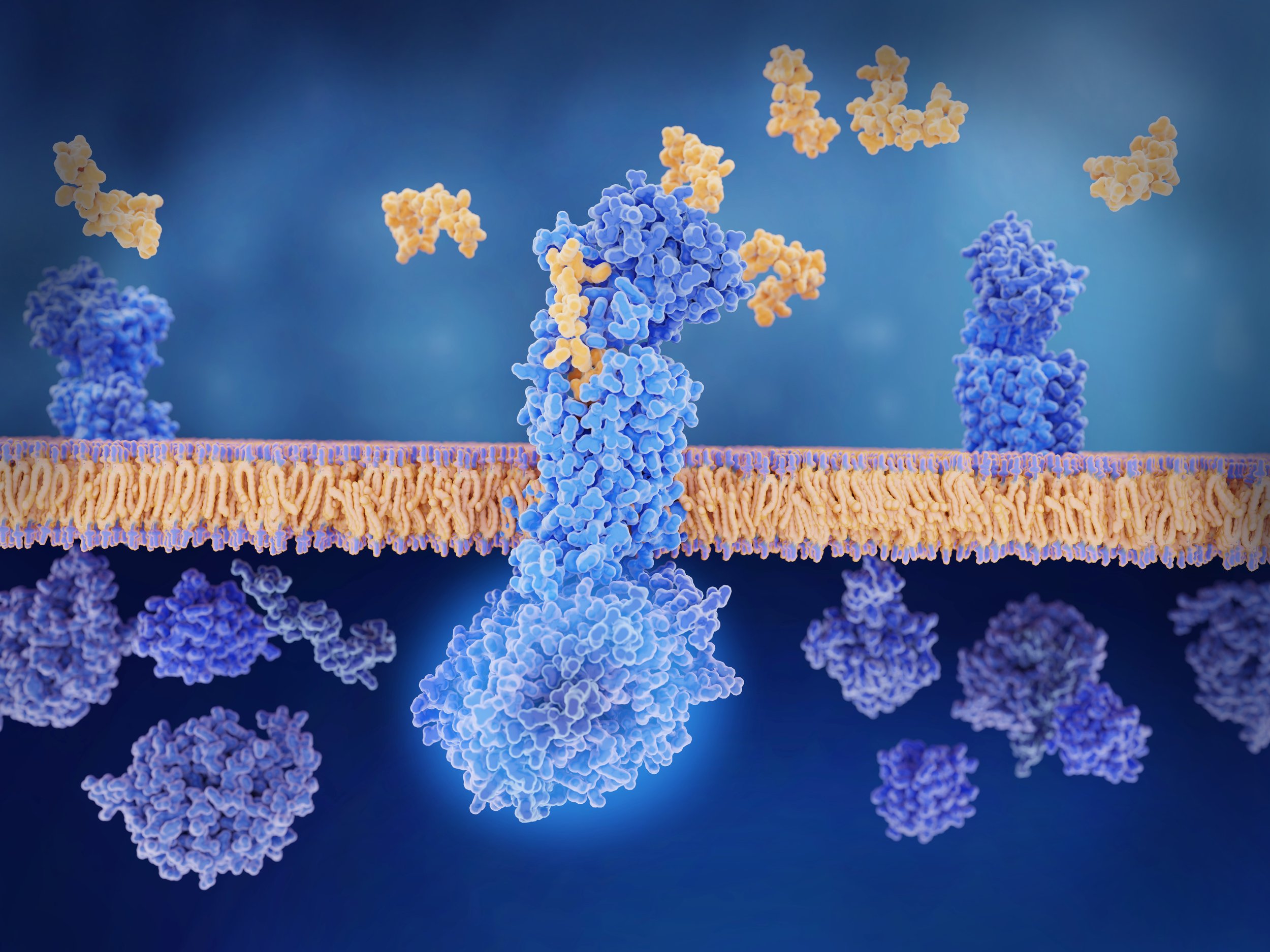
The approach to prevention therapy for migraine took an interesting and positive turn in the U.S. with the emergence of erenumab (Aimovig) in May 2018. As with the three other medications of this class which rapidly followed, Aimovig is a monoclonal antibody protein molecule that targets calcitonin gene-related peptide (CGRP). Whereas Aimovig is a human protein, galcanezumab/Emgality, fremanezumab/Ajovy and epitenzumab (Vyepti) are “humanized” mABs made from small parts of mouse proteins attached to human proteins. Because of their relatively large size the mABs are believed to lack the capacity to pass through the “blood brain barrier” from the bloodstream into the brain and so must exert their therapeutic action in the peripheral portion of the migraine circuit and at the trigeminovascular junction specifically (see “Introduction to Migraine” in this issue for a diagram of the central and peripheral nervous system components of the migraine circuit).
Calcitonin gene-related peptide is a potent vasodilator, and cranial arteries bombarded by CGRP will expand and leak proteins that promote inflammation in the pain-sensitive areas surrounding those arteries. All of the anti-CGRP Mabs “rheostat down” the sensitivity of the migraine circuit by either disabling CGRP directly or by blocking its target receptor. By either mechanism, this protein which plays such a major role in producing migraine headache is prevented from docking with its receptor and exerting its painful effect. This results in a less sensitive/more “stable” migraine circuit that is less inclined to generate a headache.
Again, there are 4 mABs currently indicated and available for migraine prevention. Three (Aimovig, Emgality, Ajovy) are self-injected subcutaneously (under the skin) once-monthly* with devices similar to an epinephrine autoinjector (EpiPen). Vyepti is administered intravenously every 3 months. Is one anti-CGRP mAB better than another? Unclear. All appear to be quite safe. None requires metabolism by the liver, and in part due to this the mABs do not interact with other medications and seldom produce side effects. Each is effective in treating both episodic and chronic migraine. Each is effective in a high percentage of patients, and in patients destined to respond the evidence of a positive response may emerge within the first few weeks following initial administration.
[*Ajovy also may be self-administered as a “triple-dose injection” every 3 months]
Frequently asked questions
But which is the best?
As there have been no scientifically rigorous head-to-head comparator trials, we simply do not have enough information available to offer an evidence-based response.
If I try one and see no improvement in my migraine, is there any point in trying another?
Just as with the triptans, therapeutic response to the mABs appears to be somewhat idiosyncratic: the same mAB that provides no relief to one migraineur will be proclaimed a “lifesaver” by another. And for you, the individual migraineur, there is some evidence to suggest that failure to respond to one mAB does not necessarily predict you will fail to respond to another. Aimovig’s mechanism of action (CGRP receptor blocker) differs somewhat from that of Emgality and Ajovy (CGRP “disablers”), and although solid evidence to support this management approach is lacking, it would seem logical to favor a switch to Aimovig if either Emgality or Ajovy is tried first and fails to help…or to either Emgality or Ajovy if initial treatment with Aimovig is ineffective.
What about Vyepti?
Isn’t an intravenously administered medication more powerful and more likely to be effective than one that is subcutaneously self-administered? Again, we just don’t know. What we do know about Vyepti is that it is “100% bioavailable” (ie, thanks to its intravenous route of administration, 100% of the medication administered enters the bloodstream and, presumably, then reaches its therapeutic target in the migraine circuit). We also know that in responders to Vyepti a positive response may occur quite quickly, even as early as the first day following initial administration. But is it better than the other 3 Mabs? Is it inclined to “work” even when the other mABs have failed? We don’t know.
Although without data from studies directly comparing one mAB to another it’s impossible to make any claim for one being “the best”, from their respective performances in large-scale clinical research trials and subsequently in clinical practice, all 4 mABs are safe, typically well-tolerated and capable of reducing migraine burden by at least 50% in a majority of patients with either episodic or chronic migraine. While a positive response to treatment may occur as early as 1 to 2 weeks following initial administration, some patients may require 2 or even 3 administrations or, in the case of Aimovig, the higher (140 mg) dose to exhibit meaningful improvement. There is also evidence from research trials involving the mABs that continued treatment in early positive responders may produce progressively greater improvement that often exceeds a 75% reduction in migraine burden relative to the patient’s pre-treatment status.
More frequently asked questions
If I have an acute headache while I’m using a mAB for migraine prevention, can I use my usual medications to treat that headache?
In a word: yes. While, like the mABs, some medications commonly prescribed for acute migraine headache may exert an effect upon arterial blood vessels, there currently exists no evidence to suggest that co-administration of such medication - a triptan or a gepant, in particular – with a mAB will pose an increased risk of stroke, heart attack or other vascular complication.
Can I use a mAB if I’m attempting to conceive, pregnant or breastfeeding?
Especially during the 3rd trimester of pregnancy, the mABs can pass from mother to developing fetus via the placental circulation, and CGRP itself may influence placental blood flow, uterine muscle tone and blood pressure. Whether these observations, largely derived from animal experiments, imply any risk to a human mother or fetus is unknown. In the absence of human data indicating an absence of risk, the existing recommendation is to avoid anti-CGRP mAB use during pregnancy unless the benefit to the patient is felt to outweigh the as-yet unknown magnitude of risk to mother or fetus.
As for breastfeeding, it is unknown whether any of the anti-CGRP mABs are present in breast milk, and, not surprisingly, there exist no meaningful human safety data. While in general migraine prevention therapy in breastfeeding females is discouraged, the American Headache Society acknowledges that the decision whether or not to treat should be individualized and take into account any safety data available as well as the degree of potential benefit to the patient/mother.
Put simply, both in regard to pregnancy and to breastfeeding…we just don’t know. Not to treat may err on the side of overcautiousness. To treat is to accept an unknown but potential risk.
If CGRP is a potent vasodilator, won’t blocking CGRP with a mAB cause me to have constricted arteries and high blood pressure…or a heart attack…or a stroke?
Such research finding as exist, in combination with now-extensive clinical experience, appear to indicate that if the mABs pose any risk of medical complications related to their promoting arterial vasoconstriction, that risk must be exceedingly low. Even so, it is recommended that the mABs be used “with caution” in patients with high blood pressure, heart disease, peripheral vascular disease or a history of stroke. This in large part reflects the fact that individuals with vascular disorders typically were excluded from the clinical research trials that earned the anti-CGRP mABs their FDA approval, and we consequently have no scientifically rigorous safety data relevant to that sub-population.
Can a mAB be combined with another migraine prevention medicine?
There is some very preliminary evidence to suggest that in patients with chronic migraine who are receiving onbotulinumtoxinA (BotoxA) injection therapy and have had a partial positive response to that therapy, the addition of a mAB is safe and may result in yet further reduction of migraine burden. As for the oral medications most commonly prescribed for migraine prevention, there is no compelling evidence that combining an oral prevention drug with a mAB is either more effective or less safe than administering the mAB alone. What doesn’t seem to make sense is to use one of the anti-CGRP “gepants”, atogepant (Qulipta) or rimigepant (Nurtec), in combination with an anti-CGRP mAB for migraine prevention.
If the mAB I’m using is effective in preventing my headaches, how long will I need to continue treatment?
An excellent question…and one long debated by medical providers who specialize in headache medicine. Migraine’s intrinsic variability, with improvement often occurring spontaneously in the absence of any treatment intervention, would seem enough to make one skeptical of “forever” prevention therapy. Furthermore, there are studies published in the peer-reviewed medical literature suggesting that, once their migraine is sufficiently stabilized, patients may discontinue prevention therapy and continue to enjoy a low migraine burden for periods extending up to years. But how long does it take to be “sufficiently stabilized”? 6 months? a year? more? There is as yet no simple answer, and if/when an answer is forthcoming, it may well depend on the specific therapy involved, the specific biology of the individual migraineur or both factors.
Will my medical insurance cover the cost of treatment with a mAB?
Again, no easy answer. Some insurers require the patient to have tried and failed up to 3 generic oral medications commonly prescribed for migraine prevention before authorization for coverage of mAB therapy will be provided. Others specify that only 1 or 2 of the mABs are “on formulary”, and many migraine patients delighted with their response to, say, Aimovig are dismayed to find that their insurer has declared the medication to be “non-formulary” and has mandated a switch to another mAB which the affected patient may find less effective. Generally, however, with some patience, the assistance of a sympathetic medical provider who will serve as your advocate in the “prior authorization” process and a boost from patient assistance programs offered by the various anti-CGRP mAB manufacturers, initiation of mAB therapy for migraine prevention can be accomplished…and at an acceptable cost to you.